the electrostatic attraction that binds oppositely charged ions together
Properties same melting and boiling points are a measure of how solid the attractive forces are between individual atoms or molecules. (We call these interunit forces – forcesbetweenmolecules, as opposed to intramolecular forces – forces within a molecule. )
It all flows from this worldwide principle: atomic number 3 bonds suit more polarized, the charges on the atoms become greater, which leads to greater intermolecular attractions, which leads to higher boiling points.
Thither are four major classes of interactions 'tween molecules and they are entirely divers manifestations of "opposite charges attract".
Now available – Download this awesome (free) 3-Page release on how to solve common boiling bespeak problems. With 10 examples of solved problems! (Also contains all the samara points discussed in this post)
MOC_Boiling_Point_Handout (PDF)
The four Key intermolecular forces are as follows:
Ionic bonds > Hydrogen soldering > New wave der Waals dipole-dipole interactions > Van der Waals dispersion forces.
Let's take them individually, from strongest to weakest.
Table of Table of contents
- Ionic Forces
- Atomic number 1 Bonding
- Vanguard Der Waals Dipole-Dipole Interactions
- Johannes Diderik van der Waals Dispersion Forces ("London forces")
- Bottom Line
1. Ionic forces
Ionic are interactions between charged atoms or molecules ("ions"). Charged ions, much as Sodium(+) , Li(+), and Ca(2+), are termed cations. Negatively charged ions, such American Samoa Cl(–), Br(–), HO(–) are called anions (I always got this straight through remembering that the "N" in "Anion" stood for "Electronegative") The attractive forces between oppositely charged ions is described aside Coulomb's Law, in which the force increases with charge and decreases as the distance between these ions is increased. The highly polarized (charged) nature of ionic molecules is reflected in their high melt points (NaCl has a freezing point of 801 °C) too as in their high tide solubility (for the base metallike salts, anyway; metals that form multiple charges like to allow for residues connected your bathtub)
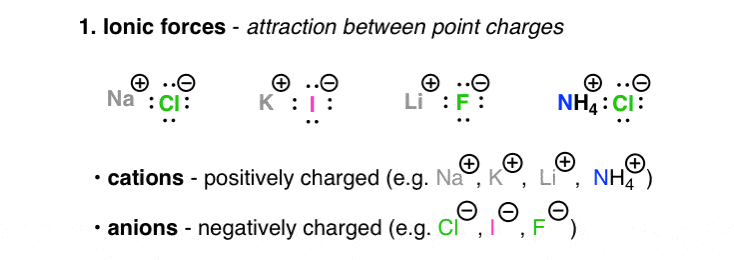
2. Hydrogen bonding
Hydrogen soldering occurs in molecules containing the highly electronegative elements F, O, or N directly bound to atomic number 1. Since H has an electronegativity of 2.2 (compare to 0.9 for Na and 0.8 for K) these bonds are non atomic number 3 polarized as purely geographic area bonds and possess roughly covalent character. However, the bond to hydrogen will still be polarized and possess a dipole.
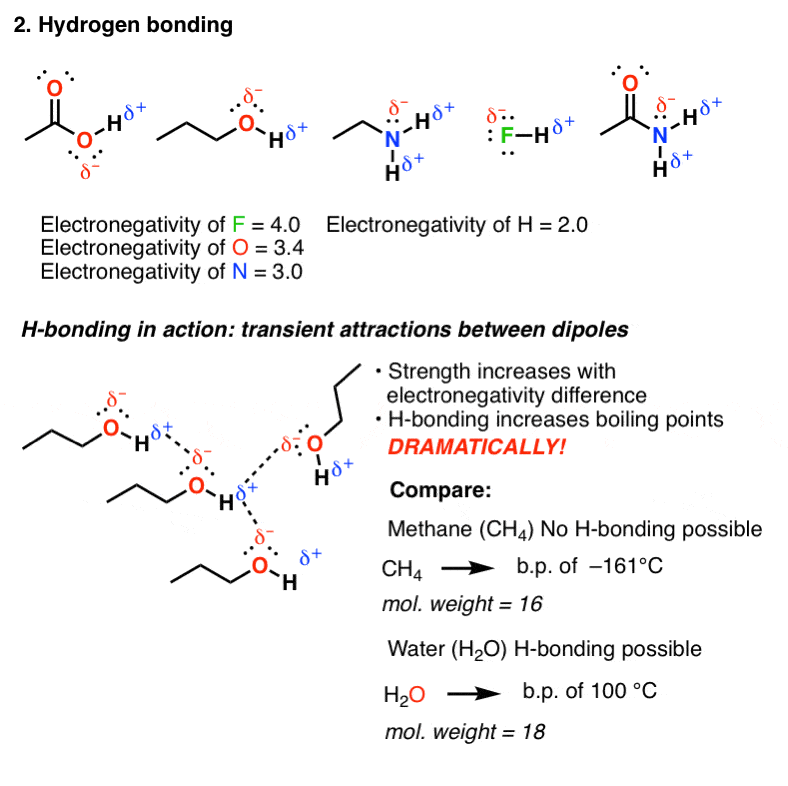
The dipole of one molecule can ordinate with the dipole from another molecule, ahead to an attractive fundamental interaction that we call hydrogen bonding. Owing to rapid molecular motion in solution, these bonds are transient (passing) but have key bond strengths ranging from (9 kJ/gram molecule (2 kcal/mol) (for NH) to about 30 kJ/mol (7 kcal) and high for HF. As you might expect, the forte of the bond increases as the negativity of the group bound to hydrogen is magnified.
Sol in a sense, HO, and NH are "sticky" – molecules containing these functional groups will tend to have higher boiling points than you would expect based happening their molecular weight.
3. Van Der Waals Dipole-Dipole Interactions
Former groups beside hydrogen can be involved in south-polar covalent soldering with strongly electronegative atoms. E.g., all of these molecules contains a dipole:
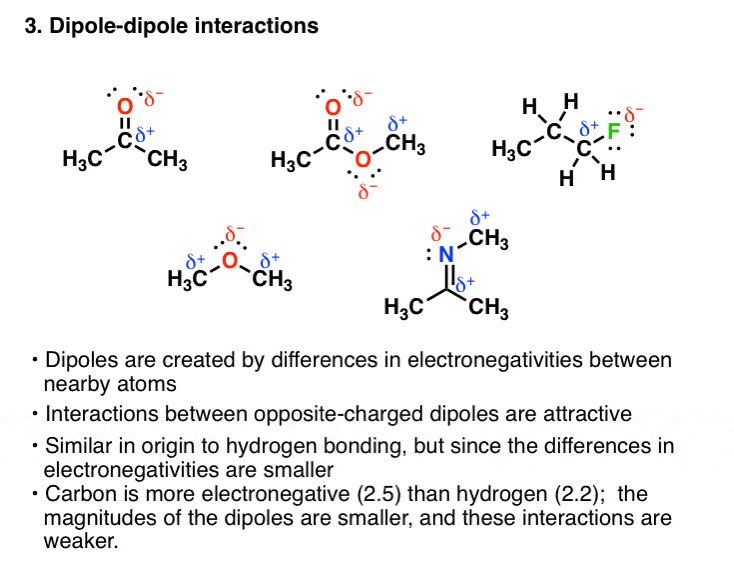
These dipoles hindquarters interact with each other in an piquant fashion, which will besides increase the boiling point. Notwithstandin since the negativity difference between carbon (electronegativity = 2.5) and the electronegative atom (such as oxygen or atomic number 7) is not as large as it is for hydrogen (electronegativity = 2.2), the polar interaction is not as noticeable. So on the average these forces tend to equal weaker than in hydrogen soldering.
4. Van der Waals Dispersion Forces ("London forces")
The weakest unit forces of all are called dispersion forces or London forces. These map the drawing card between instantaneous dipoles in a molecule. Think back all but an mote like Ar. It's an neutral gas, flop? Only if you cool it to –186 °C, you can actually concentrate it into liquid argon. The fact that it forms a liquid it means that something is holding it together. That "something" is dispersion forces. Think about the electrons in the valence trounce. On average, they're evenly dispersed. But at any given fast, there might be a mismatch between how many another electrons are on matchless pull you said it many are on the other, which can lead to an instantaneous difference in heraldic bearing.
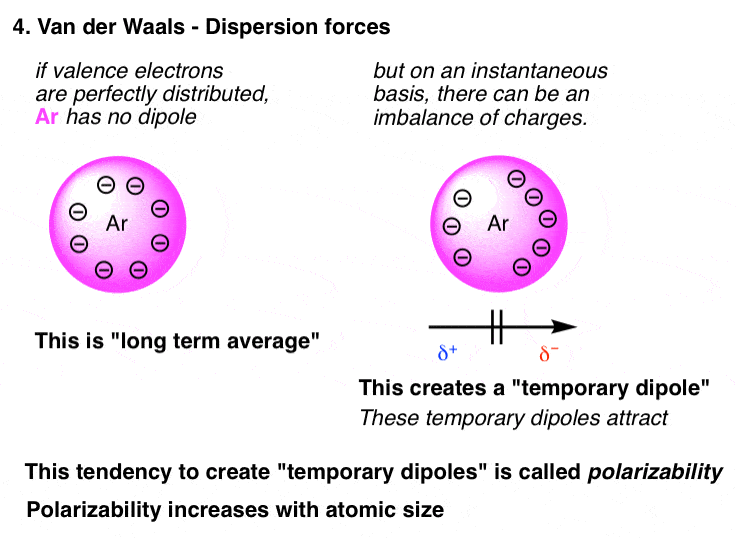
IT's a little like basketball. On the average, every player is covered one-on-same, for an tied distribution of players. Only at any given bit, you might take up a double-team situation where the distribution of players is "lumpy" (it besides means that somebody is open). In the valence shell, this "lumpiness" creates dipoles, and information technology's these dipoles which are causative intermolecular attraction.
The polarizability is the term we use to delineate how readily atoms can form these instantaneous dipoles. Polarizability increases with atomic size. That's why the stewing point of Ar (–186 °C) is such high than the boiling point of helium (–272 °C). Past the same doctrine of analogy, the boiling point of iodine, (I-I, 184 °C) is much higher than the boiling point of fluorine (F-F, –188°C).
For hydrocarbons and other non-polar molecules which deficiency strong dipoles, these dispersal forces are in truth the only piquant forces between molecules. Since the dipoles are weak and transient, they depend on inter-group communication between molecules – which means that the forces increment with shallow area. A microscopic molecule like methane has very weak intermolecular forces, and has a low boiling point. However, as molecular weight increases, boiling point also goes up. That's because the surface over which these forces can operate has increased. Therefore, dispersion forces increase with accretionary relative molecular mass. Individually, each interaction isn't worth a lot, but if collectively, these forces can be extremely significant. How can a gecko lounge lizard walk on walls? Feel at its feet.
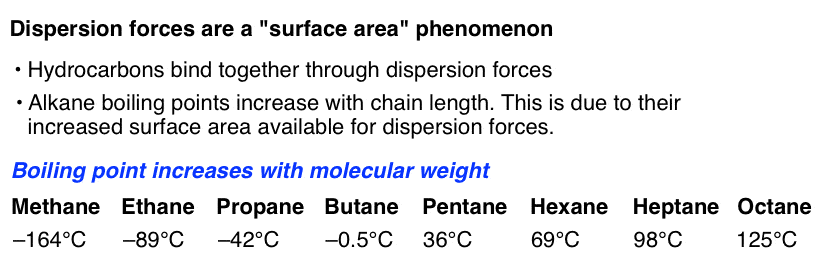
[Determining trends for hydrocarbons derriere get a little bit tricky depending on the exact structure – symmetry besides plays a role in stewing points and melting points. I talked about this in detail previously.]
5. Bottom Line
- Boiling points are a measure of intermolecular forces.
- The intermolecular forces increase with increasing polarization of bonds.
- The strength of intermolecular forces (and therefore impact happening boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion
- Stewing point increases with molecular weight, and with aboveground area.
For another treatment of these principles see Chemguide
Admonisher – don't block the rid of boil contemplate guide (Contains all the primal points discussed in this post)
MOC_Boiling_Point_Handout (PDF)
the electrostatic attraction that binds oppositely charged ions together
Source: https://www.masterorganicchemistry.com/2010/10/01/how-intermolecular-forces-affect-boiling-points/
Posting Komentar untuk "the electrostatic attraction that binds oppositely charged ions together"